IMI EHDEN presents their online roadshow : a series of 3 webinars focussed on
‘The Role of Health Data in the Post Covid-19 Era’

EHDEN Roadshow October 2020
Public report
Executive Summary
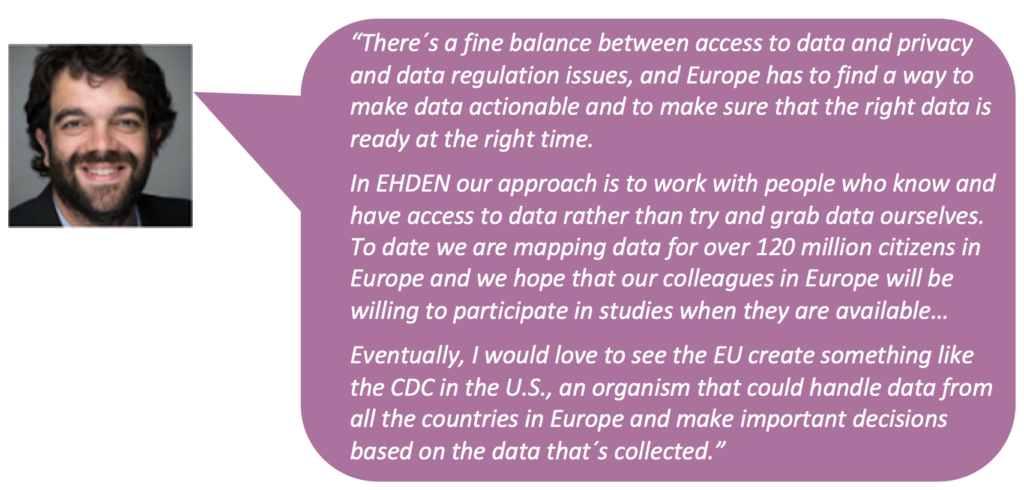
The European Health Data and Evidence Network (EHDEN) is a pan-European public-private partnership of 22 organisations, led by Erasmus Medical Center and Janssen Pharmaceutica, whose mission is to make large-scale analysis of health data in Europe a reality. Under the aegis of the EU´s Innovative Medicines Initiative and scheduled to run until 2024, it is spearheading the development of a federated data network standardised to a common data model. This greatly facilitates the sharing of health data and research regardless of the organisation or country they originate from.
In October 2020, EHDEN held its first annual roadshow to highlight accomplishments to date, share perspectives on where the health research ecosystem is headed, and map in broad strokes its vision for the remaining four years of its mandate. Due to the ongoing COVID-19 pandemic, the event took place virtually, but that did not impede it from bringing together some of the most passionate defenders of future-forward ideas and approaches to real-world evidence collection, data governance, data ownership, health technology assessment, health data science, patient and stakeholder engagement, biopharma´s future, and public policy, both within and beyond the boundaries of the EHDEN project.
This report presents the main takeaways that emerged from those discussions, which can be heard in their entirety across three individual podcasts (podcast 1, podcast 2, and podcast 3) and three roundtable-style webcasts (webinar 1, webinar 2, and webinar 3). The conversations centred around the following themes:
- The value of real-world data for assessing treatment effectiveness
- The research potential of standardising health data across Europe and mapping them to a common data model that enables systematic analysis of disparate observational databases
- The role of open source software for building out a data strategy for secondary use of health data, while preserving data ownership through built-in privacy mechanisms
- EHDEN´s role as an accelerator of these processes by creating a “marketplace” for data partners and data translators to connect
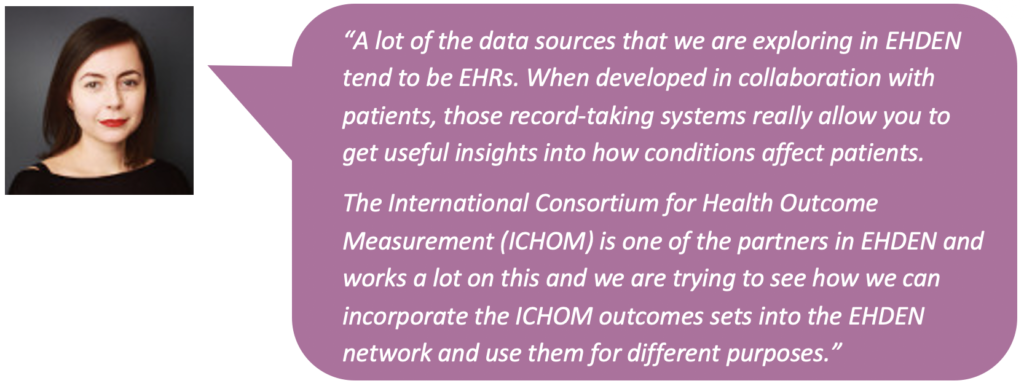
- The role of early patient involvement and participation in outcomes research
- New ways to think about the value of health data, data access requirements, and marketing authorisation as a way to propel open science research forward
Click on the session titles below for a summary report per session of click here to download the entire report
In conclusion, the EHDEN Virtual Roadshow provided a robust platform for a broad range of experts to share thoughts on Europe’s health research ecosystem. The collaboration that was in full force during the event is a critical aspect of a federated data network and is key to fostering a research community that more smartly manages and shares real-world data.
This webinar series is supported by: